
These can often be reduced or eliminated by increasing your annealing temperature. More often, the non-specific bands will mostly be smaller than your desired product. Just don't give the polymerase enough time to make a 2-kb product if you know you only want a 1-kb product. If your goal is, say, a 1kb band, and you're getting a non-specific 2-kb band, that means your elongation step is probably twice as long as it needs to be. Ignore this second option for the time being.Īre some of the non-specific bands you're seeing longer than your actual desired band? If so, decrease your elongation time. Less often, but more problematic, you may have additional non-specific binding sites within the desired product itself.
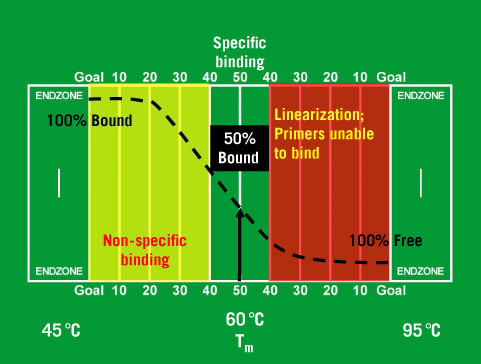
If your template DNA is some sort of genomic DNA, the non-specific bands probably represent other spots in the genome where the primers are a close-enough match to produce a product. Double check your reference sequence with the 1000 genome project or alternatives to avoid SNPs hotspots. Human samples commonly have SNPs, particularly so for exons. Whenever possible, aim for a 3 hydrogen bond primer on that 3' site. The last nucleotide in your primer sequence is critical. G quadruplex are a catastrophe for DNA, as it leads to secondary structures. Use HotStart polymerases for hard to amplify reactions. This once isnt directly a problem with your primer rather the polymerase you use with it. Fairly common to have a primer binding to multiple sites, greatly reducing efficiency.
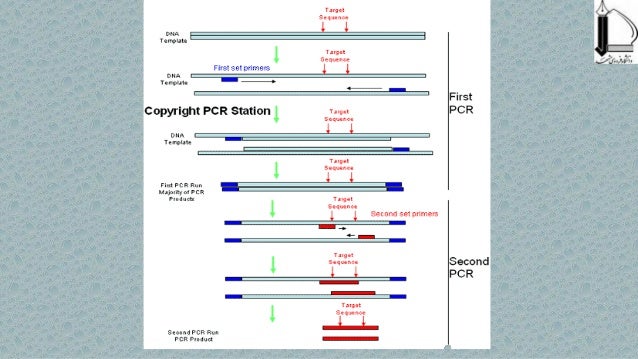
If it is genomic DNA/ cDNA library, double check off target binding of your primers using blast. Worse, at >50b, about 50% of your primer mix is not the sequence you ordered, if you ordered desalt that is. When you order a primer you actually order a pool of synthesis products that, when you reach >30b, start to take a significant proportion of your primer.
#Alternative to touchdown pcr protocol upgrade
For primers >40b upgrade cleaning method from your supplier, desalt wont cut it. Forget annealing temp, if you have issues with your PCR thats most likely not the issue.


 0 kommentar(er)
0 kommentar(er)
